Earthworms, Enzymes, and Circulation: The Science Behind Lumbrokinase
Blood clotting is essential for healing and other important functions throughout the body, but when the process goes unchecked, it may have long-term implications for cardiovascular health.
When you get injured, your body initiates a cascade of enzymatic reactions that converts fibrinogen into fibrin. Fibrin is a tough, fibrous protein that forms the structural mesh of a blood clot. This insoluble fibrin mesh traps platelets and blood cells, sealing the injury and stopping bleeding.
While clot formation is critical in the acute stage of tissue repair, excess fibrin or impaired breakdown of clots (fibrinolysis) can contribute to chronic inflammation, poor circulation, and even serious cardiovascular events.1
Clot Formation and Fibrinolysis: A Delicate Balance
Healthy circulation depends on the balance between coagulation (clot formation) and fibrinolysis (clot breakdown).
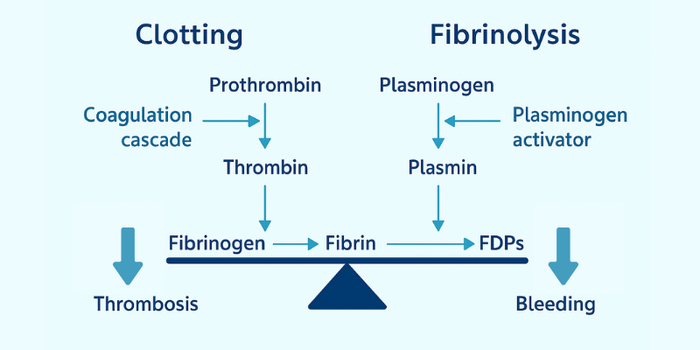
When bleeding occurs, prothrombin is activated to thrombin, which converts fibrinogen into fibrin to form a clot. Later, plasminogen is activated to plasmin, often by tissue plasminogen activator (tPA), to break down the clot into fibrin degradation products (FDPs). This is how the body removes clots once they’ve served their purpose. These two processes must remain in a healthy balance.1
Understanding Hypercoagulability
A hypercoagulable state occurs when blood is more prone to clotting than it should be, even in the absence of injury. Many factors can contribute to the blood becoming “too thick,” including:2-8
- Chronic inflammation: Pro-inflammatory cytokines like IL-6 and TNF-α stimulate the overproduction of clotting factors and fibrinogen, promoting a hypercoagulable state.
- Impaired fibrinolysis: Fibrin degradation is often hindered by elevated levels of plasminogen activator inhibitor-1 (PAI-1), which inhibits tPA. PAI-1 is commonly elevated in insulin resistance, obesity, type 2 diabetes, hypertriglyceridemia, aging, and chronic inflammation.
- Sluggish circulation: Reduced blood flow due to physical inactivity or vascular dysfunction can cause blood to pool and promote clot formation.
- Oxidative stress and endothelial dysfunction: Oxidative damage to blood vessels impairs nitric oxide production, activates platelets, oxidizes LDL, and promotes adhesion molecule expression, all of which promote clot formation.
- Hormonal influences: Estrogen dominance, oral contraceptive use, and dysregulated cortisol levels can increase clotting risk.
- Infections: Infections can trigger coagulation as part of the immune response. For example, Staphylococcus aureus promotes fibrin formation as a protective biofilm strategy in endocarditis.
Fibrinolytic Enzymes: Natural Tools for Healthy Coagulation
Fibrinolytic enzymes have been shown to support the body’s ability to break down excess fibrin. Found as supplements, their use is gaining popularity for promoting cardiovascular health and maintaining normal blood viscosity.
Lumbrokinase is a fibrinolytic enzyme derived from earthworms, with two main mechanisms of action:9-10
- Direct fibrinolysis: Supports healthy breakdown of blood clots.
- Indirect fibrinolysis: Promotes plasminogen-to-plasmin conversion, aiding the natural fibrin degradation process.
It also promotes tPA activity, further supporting plasminogen-to-plasmin conversion.
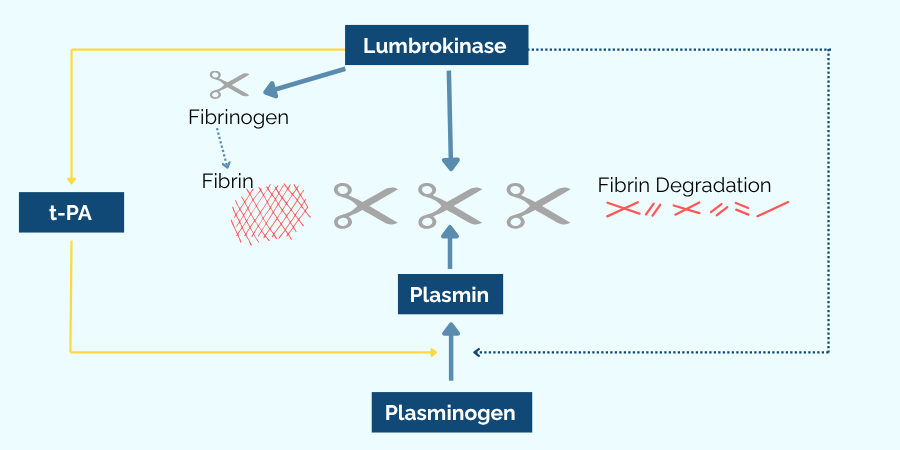
Unlike broader-acting enzymes, lumbrokinase acts selectively in the presence of fibrin, which may reduce the likelihood of interfering with normal clotting processes.10-12
Lumbrokinase vs. Nattokinase
In contrast, nattokinase, an enzyme derived from Bacillus subtilis during the fermentation of soybeans, has broader activity. In addition to supporting fibrinolysis, it may also degrade thrombin, an enzyme involved in clot formation. Some evidence suggests this activity raises the potential for excessive bleeding, impacting clotting labs like PT, PTT, and INR, effects not typically seen with lumbrokinase.
In other words, lumbrokinase is only active in the presence of pre-existing clots, thus preserving healthy physiologic clotting, while nattokinase may disrupt both existing clots and the formation of new ones. This makes lumbrokinase a more targeted and potentially safer option for long-term or sensitive use.1,11-14
Other enzymes, such as serratiopeptidase and bromelain, also exhibit mild fibrinolytic activity, but their actions are less specific. These enzymes have non-specific proteolytic effects, meaning they break down a wider range of proteins rather than targeting fibrin alone.15-17
Not all fibrinolytic enzymes are created equal. It is vital to select an enzyme that best meets the specific needs of the individual.
Decoding Enzyme Activity
Understanding the differences between International Units (IUs), Fibrinolytic Units (FUs), and Lumbrokinase Units (LKUs) is essential for interpreting product labels and evaluating potency.
Enzymes are measured not just by weight (in milligrams) but, more importantly, by activity, or how effective they are at catalyzing a specific reaction under defined conditions. Fibrinolytic enzymes are quantified using several distinct units of measurement, each reflecting different assay methodologies and standards. Here’s how to interpret some of the most common units:
- International Units (IUs): A measurement used when enzyme activity is standardized globally. For each substance, 1 IU corresponds to a defined amount of enzymatic activity, determined by international consensus. This allows for cross-comparison between agents.18
- Fibrinolytic Units (FUs): A general term used to quantify the fibrin-degrading activity of an enzyme in a test tube. It is typically established by comparing the activity of the enzyme to a standard clot degradation rate. However, the exact testing methodology can vary between labs, limiting direct comparability unless all samples are tested using the same methodology.19
- Lumbrokinase Units (LKUs): This unit is specific to lumbrokinase and is used to reflect the activity of this group of enzymes. LKUs are not standardized internationally and are typically defined by individual companies or laboratories using proprietary or in-house reference standards. It is thus not directly interchangeable with IUs or other units of measurement, and not a reliable measurement to reference when comparing enzymes from different suppliers
There are several other units of measurement used depending on the type of enzyme and activity being measured. Notably, while we may see weight-based labeling in milligrams (mg), this is often misleading, as weight does not indicate activity. Two products of the same weight may have vastly different fibrinolytic potency depending on purity and manufacturing specifications.20
Limitations and Nuance
While lab testing provides important insights, real-world efficacy and comparison of fibrinolytic enzymes may be impacted by several variables
- Formulation factors: Enteric coating, enzyme stability, and delivery format significantly affect how well the enzyme survives digestion and reaches the bloodstream intact. 20
- Manufacturing quality: Purity, potency, and consistency can vary widely between brands.
- Lack of standardization: Different measurement units (IU, FU, LKU) are not interchangeable, making product comparisons challenging.
Always evaluate fibrinolytic supplements based on activity units, not gross weight. Two products with identical weights may differ dramatically in potency. If comparing multiple products, you must use a consistent assay or unit across all samples to assess potency.21
How Fibrinolytic Enzymes are Applied Clinically
Emerging clinical evidence supports diverse applications for enzymes like lumbrokinase. In one study, long-term use was associated with support for circulatory and neurological function.22 Preclinical data also suggest it may influence fibroblast activity and tissue adhesion.23
Functional medicine providers often explore fibrinolytic enzyme use for supporting healthy coagulation and even healthy uterine blood flow.24-26
While further research is warranted, current evidence shows promising potential for lumbrokinase in supporting healthy circulation. As with any dietary supplement, safety should be evaluated on an individual basis by a qualified healthcare practitioner, especially for patients using anticoagulants or other medications that impact clotting.
Navigating the Enzyme Landscape
Fibrinolytic enzymes represent a promising frontier in natural cardiovascular and coagulation support. Knowing the source, mechanisms, and most importantly, how enzymatic activity is measured can help determine whether a product is effective and appropriate for clinical use.
References
- Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. SpringerLink. 2017;82:405-456. doi:https://doi.org/10.1007/978-3-319-49674-0_13
- Balagopal P (Babu), de Ferranti SD, Cook S, et al. Nontraditional Risk Factors and Biomarkers for Cardiovascular Disease: Mechanistic, Research, and Clinical Considerations for Youth. Circulation. 2011;123(23):2749-2769. doi:https://doi.org/10.1161/cir.0b013e31821c7c64
- Altalhi R, Pechlivani N, Ajjan RA. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. International Journal of Molecular Sciences. 2021;22(6):3170. doi:https://doi.org/10.3390/ijms22063170
- Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Frontiers in Bioscience. 2007;12(8-12):2957. doi:https://doi.org/10.2741/2285
- Scioli MG, Storti G, D’Amico F, et al. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. Journal of Clinical Medicine. 2020;9(6):1995. doi:https://doi.org/10.3390/jcm9061995
- Trigg DE, Wood MG, Kouides PA, Kadir RA. Hormonal Influences on Hemostasis in Women. Seminars in Thrombosis and Hemostasis. 2011;37(01):077-086. doi:https://doi.org/10.1055/s-0030-1270074
- Kell DB, Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integrative Biology. 2014;7(1):24-52. doi:https://doi.org/10.1039/c4ib00173g
- Oukrich S, Hong J, Leon-Grooters M, et al. Early fibrin biofilm development in cardiovascular infections. Biofilm. 2025;9:100261. doi:https://doi.org/10.1016/j.bioflm.2025.100261
- Wang KY, Tull L, Cooper E, Wang N, Liu D. Recombinant Protein Production of Earthworm Lumbrokinase for Potential Antithrombotic Application. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1-8. doi:https://doi.org/10.1155/2013/783971
- Li G, Wang KY, Li D, Wang N, Liu D. Cloning, Expression and Characterization of a Gene from Earthworm Eisenia fetida Encoding a Blood-Clot Dissolving Protein. Pratap J, ed. PLoS ONE. 2012;7(12):e53110. doi:https://doi.org/10.1371/journal.pone.0053110
- Ji H, Wang L, Bi H, et al. Mechanisms of lumbrokinase in protection of cerebral ischemia. European Journal of Pharmacology. 2008;590(1-3):281-289. doi:https://doi.org/10.1016/j.ejphar.2008.05.037
- Tjandrawinata R, Yunaidi D, Susanto L. The Safety and Tolerability of Lumbrokinase DLBS1033 in Healthy Adult Subjects. Drug Research. 2016;66(06):293-299. doi:https://doi.org/10.1055/s-0035-1569406
- Kurosawa Y, Nirengi S, Homma T, et al. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Scientific Reports. 2015;5(1):11601. doi:https://doi.org/10.1038/srep11601
- Wang Y, Wang H, Zhang Y, Xu F, Wang J, Zhang F. Stepwise Strategy to Identify Thrombin as a Hydrolytic Substrate for Nattokinase. Journal of chemical information and modeling. 2022;62(22):5780-5793. doi:https://doi.org/10.1021/acs.jcim.2c00978
- Nair SR, C SDevi. Serratiopeptidase: An integrated View of Multifaceted Therapeutic Enzyme. Biomolecules. 2022;12(10):1468. doi:https://doi.org/10.3390/biom12101468
- Bhagat S, Agarwal M, Roy V. Serratiopeptidase: A systematic review of the existing evidence. International Journal of Surgery. 2013;11(3):209-217. doi:https://doi.org/10.1016/j.ijsu.2013.01.010
- Varilla C, Marcone M, Paiva L, Baptista J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods. 2021;10(10):2249. doi:https://doi.org/10.3390/foods10102249
- National Cancer Institute at the National Institutes of Health. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/international-unit. www.cancer.gov. Published February 2, 2011. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/international-unit
- Kotb E. Activity assessment of microbial fibrinolytic enzymes. Applied Microbiology and Biotechnology. 2013;97(15):6647-6665. doi:https://doi.org/10.1007/s00253-013-5052-1
- Tjandrawinata R, Trisina J, Rahayu P, Prasetya L, Hanafiah A, Rachmawati H. Bioactive protein fraction DLBS1033 containing lumbrokinase isolated from Lumbricus rubellus: ex vivo, in vivo, and pharmaceutic studies. Drug Design, Development and Therapy. Published online September 2014:1585. doi:https://doi.org/10.2147/dddt.s66007
- Thelwell C. Biological Standards for Potency Assignment to Fibrinolytic Agents Used in Thrombolytic Therapy. Seminars in Thrombosis and Hemostasis. 2014;40(02):205-213. doi:https://doi.org/10.1055/s-0033-1364188
- Cao Y, Zhang X, Wang W, et al. Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: results from a multicenter, randomized, parallel-group and controlled clinical trial. Chinese Medical Journal. 2013;126(21):4060. doi:https://doi.org/10.3760/cma.j.issn.0366-6999.20131332
- Nguyen QTT, Rhee H, Kim M, Lee MY, Lee EJ. Lumbrokinase, a Fibrinolytic Enzyme, Prevents Intra-Abdominal Adhesion by Inhibiting the Migrative and Adhesive Activities of Fibroblast via Attenuation of the AP-1/ICAM-1 Signaling Pathway. BioMed Research International. 2023;2023:4050730. doi:https://doi.org/10.1155/2023/4050730
- J. Massimo Nunes, Kell DB, Pretorius E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses. Blood Rev. 2023;60:101075-101075. doi:https://doi.org/10.1016/j.blre.2023.101075
- Senst B, Tadi P, Basit H, Jan A. Hypercoagulability. PubMed. Published August 22, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538251/
- Mancini B, Kaleta F, Zimmerman E, Uqdah H, Elnaggar A, Pfeiffer M. Aortic Thrombus in Severe Disseminated Lyme Disease. Annals of Internal Medicine: Clinical Cases. 2024;3(5). doi:https://doi.org/10.7326/aimcc.2023.0843